
What is Struvite?
Struvite is a phosphate mineral with the formula: NH4MgPO4.6H2O. It crystallises as in an orthorhombic structure with the appearance of white to yellowish or brownish-white pyramidal crystals.
Its Mohs hardness is fairly soft at 1.5 to 2. And has a low specific gravity of 1.7. It is sparingly soluble in neutral and alkaline conditions, but readily soluble in acid.

Formation of Struvite?
The reaction to form struvite is simple, however the process is complex and depends on several things, including:
- The ionic strength
- pH
- alkalinity
- temperature of the waste stream
The struvite solubility limit curve below can be used to determine if struvite formation is likely to occur:
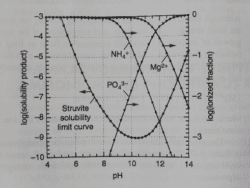
There are several calculations involved to determine if the formation of struvite will occur and these are then plotted on the curves.
Struvite Formation Conditions
- 1:1:1 concentration ratio in Mg, NH4, PO4 to form NH4MgPO4.6H2O in the liquid interface not in the solids
- High retention times (e.g. slow moving liquid in long pipes)
- High temperature conditions and/or (e.g. warm liquid in half full transfer lines)
- High pressure conditions (e.g. along U-bends, S-bends or continuous piping changing in diameter, where solids can accumulate)
- Evaporation points downstream from high speed and/or high temperature units (e.g. centrifuges or pumps)
Crystallisation will not occur unless all conditions are available
In summary, the formation of struvite involves the right combination of conditions and ions present to form. So, what else could be forming?
Other precipitates in WWT and AD plants?
- Calcium carbonate (scale formation)
- Vivianite scaling
- Variscite
It is important to note that both increase in pressure as well as pH can result in the formation of a build-up of calcium carbonate and/or struvite provided the other conditions for these scale formations are also present.
Determining which one is present?
A simple analytical elemental analysis such as Inductively Coupled Plasma (ICP) will not be sufficient to identify which of the different minerals/scales are present as this simply gives an idea of how much and which elements are present.
A hardness test will distinguish between struvite and calcium carbonate as their Mohrs hardness is different. However, struvite and vivianite will have similar hardness values. Microscopy can also be used to distinguish between struvite and calcium carbonate.
Analytical techniques such as X-ray diffraction (XRD) and Raman Spectroscopy will give you a better understanding of how the elements are structured and their bonding arrangements.
With each one being unique.
OMEX offer full technical support in their fully equipped laboratory, in order to recommend process optimisation solutions.



